3D printed device resolves lack of medical equipment during Coronavirus crisis!
According to our legit source, 3D printed device should help resolve the lack of medical equipment during Coronavirus crisis. Discover more details below!

3D printed device resolve lack of medical equipment during Coronavirus crisis!
The non-profit health company – Prisma Health, which manages 18 hospitals in southern California – announced the approval of its emergency equipment from the FDA. It is a ventilator expansion allowing a single ventilator to be used for a maximum of four victims withal. By doing this, Stephanie Hendrixson and partner has researched and developed emergency surrounding devices to “separate” cooler; sharing for multiple patients taking at the same time.
It is a movement that controversy because it doesn’t use in a typical medical situation. The new equipment, dubbed “VESper” by company, is a Y-shape tube that allows viruses and bacterium to filter, the firm said. Moreover, Prisma Health produces mass this device throughout 3D printed technology. The announcement from Prisma Health reveals that HP is their production partner. HP is a developer of 3D printing technologies mainly aim at manufacturing.
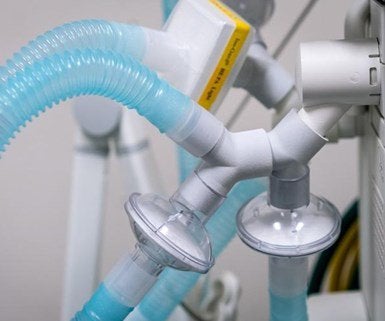
The Prisma announcement quotes HP’s Digital Manufacturing Network. It is a dispersive network to connect the 3D machines in different places; like resources can allow production to scale up quickly. However, this tech can provide to produce the devices on 3D printers outside this network. The hospitals can register to get these specifications at prismahealth.org/VESper. Some roles for 3D printing technology to help fight Coronavirus. Firstly, Ford is making a transparent shield model of face for medical workers and first responders, with a target of over 100, 000 per week. Second, a Silicon Valley 3D printing firm is working on a face shield and cotton swabs; writes Forbes.
What’s needed?
The medical equipment companies need to begin making now to testify 3D printing ingredients to reserve when the next critic is happening, says Beuth.









